 |
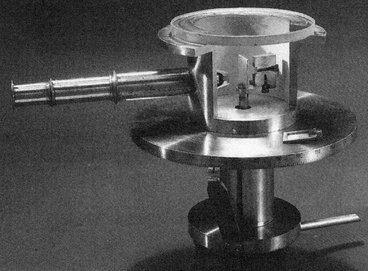
| Replica of an apparatus used by Geiger and Marsden |
Gold foil wasn’t something Rutherford or Geiger and Marsden just randomly had in their lab — its unique properties had already made it a favorite tool for experimental physicists long before 1909. Here’s the story up to that point:
1. Gold’s Unusual Physical Properties
-
Malleability: Gold is the most malleable element known. By the 18th and 19th centuries, craftsmen could hammer it into sheets just a few hundred nanometers thick.
-
Durability: Unlike many metals, gold doesn’t corrode or oxidize in air. A sheet of gold could be exposed for years without degradation, making it ideal for precision optics and experiments.
These two qualities made gold foil attractive to early physicists.
2. Use in Early Optical and Radiation Experiments (1800s)
-
Thin metal films for optics: By the mid-1800s, physicists (and chemists) were using extremely thin gold leaf to study optical transparency of metals — how light transmits through thin sheets.
-
Electrostatics: Gold leaf had been used in gold-leaf electroscopes since the late 18th century (Abraham Bennet, ~1780s). In these devices, strips of gold foil diverged when charged, providing a sensitive way to detect static electricity.
-
Early radiation experiments: By the late 19th century, gold foil was being used to stop or attenuate cathode rays (electrons) and X-rays. Its thinness allowed experimenters to study how much penetration various forms of radiation had.
3. Right Before Rutherford (1890s–1900s)
-
Henri Becquerel (1896) and the Curies used gold foil (among other materials) to study how uranium and radium radiation was absorbed or transmitted.
-
Ernest Rutherford himself (before the scattering experiments) had used thin metal foils, including gold to study the penetration of alpha particles in matter (1899–1905). He measured ranges of alpha particles by seeing whether they passed through thin sheets.
-
Geiger (before collaborating with Rutherford) also worked with gold foils to detect ionization caused by radiation.
So, by the time of the gold foil experiment (1909), physicists were already accustomed to using gold foil as the “standard” thin, stable, uniform test barrier for radiation studies.
✅ In summary:
-
1780s → Gold leaf used in electroscopes (charge detection).
-
1800s → Thin foils studied for light transmission and electrostatics.
-
Late 1800s → Used in absorption studies of cathode rays, X-rays, and radioactivity.
-
1899–1905 → Rutherford himself used gold foils to measure alpha particle ranges.
-
1909 → Geiger and Marsden’s gold foil scattering experiment revealed the atomic nucleus.
So gold foil had a long pedigree: first in electrostatics, then in radiation absorption, before becoming the decisive material in discovering the nucleus.
